A weakened immune system has long been attributed to aging and poor lifestyle choices, but according to an October 2023 study1 In Nature Communications, a key reason for this immune system decline is dysfunction of mitochondria, the powerhouses of cells, specifically those found in T cells, a type of immune cell.
When mitochondria don’t function properly, T cells don’t have the energy they need to perform their functions, resulting in a weakened immune system. This in turn leads to a failure to prevent acute infections and chronic diseases. According to Medical Xpress:2
“…in the immune system, chronic infection and defense against tumors often lead to the phenomenon of T cell exhaustion: a process in which T lymphocytes gradually lose their function, thereby weakening their response to cancer and infection. …..
This study now shows that the depletion process is significantly affected by mitochondria. When mitochondrial respiration fails, it triggers a cascade of reactions that ultimately lead to genetic and metabolic reprogramming of T cells, a process that leads to their functional failure. “
The good news, confirmed by dedicated research, is that this decline can be reversed through treatments that target mitochondrial function.
Mitochondrial dysfunction leads to T cell exhaustion
Simply put, when your body fights an infection, immune cells called CD8+ T cells convert into cytotoxic T lymphocytes (CTL) to destroy infected cells. This transformation requires changes in gene expression, cellular structure, and energy utilization.
However, in long-term infections or cancer, T cells can become worn out or “exhausted,” losing their effectiveness. This fatigue is related to energy problems within the cells, specifically within the mitochondria. Researchers are now exploring how to resolve these energy issues to rejuvenate exhausted T cells, thereby improving cancer treatment. Bioenergetic researcher Georgi Dinkov commented on the findings:3
“Until now, the decline in immune function that occurs during aging has been explained by the simple concept of ‘wear and tear’, and when immune deficiencies develop in younger people, it has been attributed either to genetic vulnerability or lifestyle choices. Such as drinking/drugs.
In other words, to date, medicine does not seem to have a good explanation for why immune function fails in aging and disease, and what, if anything, can be done to prevent this.
Research shows that the direct cause of decreased immunity is quite simple—decreased mitochondrial function (OXPHOS). When T cells (immune cells produced by the thymus) have mitochondrial dysfunction, they must rely entirely on glycolysis to produce energy.
The energy produced by glycolysis is insufficient to support proper T cell differentiation and activity. In fact, when glycolysis is the main energy mode, a large amount of reactive oxygen species (ROS) will be produced, leading to T cell damage or even death.
Research has also shown that this mitochondrial dysfunction is a necessary and sufficient condition for immune decline (also known as T-cell ‘exhaustion’) to occur and that immune decline is reversible when mitochondrial function is restored pharmacologically. “
Glucose Metabolism 101
So, what is glycolysis for energy and why is it so harmful? All dietary carbohydrates are digested and broken down into glucose (a type of sugar). Glucose, in turn, can be metabolized (burned) into fuel through two different pathways, as shown in the diagram below.
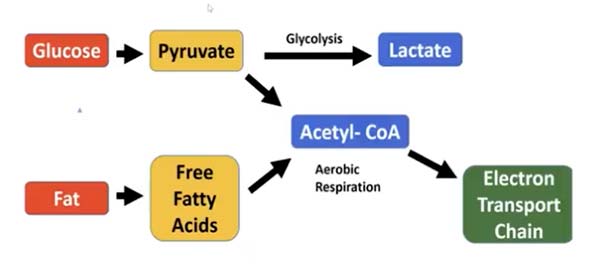
First, glucose is metabolized into pyruvate. Pyruvate can then enter the glycolytic pathway in the cell cytoplasm and produce lactate, or it can be converted to acetyl-CoA and shuttled to the mitochondrial electron transport chain.
Cancer cells are notorious for using the glycolytic pathway—when glucose metabolism in mitochondria is impaired, glucose undergoes the same pathway. Basically, this is the pathway your body uses when it reaches its limit on the amount of ATP produced in your mitochondria (which is the most efficient and least destructive way to produce energy).
Downstream hazards of glycolysis
While the glycolytic pathway is useful when you need a quick boost of energy, if this is your primary way of burning glucose, you’re in a state of continually activating stress hormones and promoting insulin resistance and diabetes, which in turn can Produces large amounts of lactic acid as a waste product to replace healthy carbon dioxide (CO2) and metabolic water.
Lactate increases reductive stress, which causes reverse electron flow in mitochondria and increases ROS to 3 to 4%, which is a 30- to 40-fold increase compared to when glucose is burned in mitochondria. Increased ROS production is responsible for T cell damage and death.
In addition, the glycolysis of each glucose molecule only produces two ATPs, which is 95% less energy than the energy produced by glucose metabolism in the mitochondria.
The devil is in the details
Now, you may have heard that sugar promotes cancer because cancer cells preferentially use glycolysis. However, it is a mistake to think that all glucose uses the glycolytic pathway. As mentioned above, glucose can also be burned in the electron transport chain of the mitochondria, which is the most efficient way to produce energy.
So when it comes to sugar-fueled cancer, it’s important to differentiate between the sources of carbohydrates. While it is technically accurate to call all carbohydrates sugars, there are fundamental differences in where the carbohydrates come from – for example, ripe whole fruit vs. starch, and whole fruit vs. refined processed sugars (ex: sucrose vs. high fructose corn syrup).
Refined sugars, as well as many starches, are common causes of endotoxin production in the gut, which can disrupt mitochondrial function and contribute to cancer metabolism, whereas fructose present in natural foods does not typically contribute to endotoxin production.
This is one of the key differences between refined sugar and ripe fruit fructose, and helps explain why refined sugar promotes cancer while natural fructose does not. So, to be clear, sugar itself is not what drives the cancer process. It actually has its roots in mitochondrial dysfunction, and fatty acid oxidation (metabolism of fat instead of glucose) is partly responsible for this dysfunction.
You want to burn glucose in the mitochondria
For a long time, I believed that fat burned “cleaner” than carbs—one of the “selling points” of keto—but then I realized we were falling behind. When glucose is burned in the mitochondria, it actually burns much more cleanly than fat.
Therefore, it’s important to maintain the correct macronutrient ratios because if the glucose you consume keeps going into glycolysis, it can fuel cancer. Also, the fat you consume ends up being stored instead of being used as fuel.
Ultimately, you want to burn glucose in the mitochondria, and the way to ensure this is to keep dietary fat intake to less than 35% of total calories. The reason is that when fat intake is too high, glucose enters glycolysis. For a more in-depth explanation of this metabolic switch, see Understanding the Randall Cycle.
If you have insulin resistance, meaning your metabolism is inflexible, the threshold may be closer to 20% or even 10%. Therefore, if you have insulin resistance, you will need to significantly reduce your fat intake until the insulin resistance resolves. Then you can increase it to 30%.
Dysfunctional T cells and cancer cells exploit glycolysis
The reason cancer cells use the glycolytic pathway is because their mitochondria are severely dysfunctional. Mitochondria are so damaged that they are unable to burn glucose. Therefore, cancer cells must rely on backup system glycolysis to survive. This is what the Warburg Effect is all about.
Likewise, when mitochondria within T cells become dysfunctional, the T cells are forced to rely on glycolysis for energy production, which is why the immune system weakens and fails.
As mentioned in the featured study, T cell exhaustion is a hallmark of cancer, which makes sense when you consider that it’s all related to mitochondrial dysfunction. Cancer develops because the mitochondria in cells become severely damaged, and as the disease progresses, mitochondria in T cells also begin to fail.
Since mitochondrial dysfunction is at the heart of it all, the most effective strategy is to use metabolic therapies to address the reason why cells are unable to oxidize (burn) sugar in the mitochondria. Once the mitochondria are repaired so that they can produce enough energy again, the cancer usually regresses and immune function returns because they no longer need to rely on glycolysis.
What are the causes of mitochondrial dysfunction?
There are four main factors responsible for mitochondrial dysfunction:
- Excessive intake of linoleic acid (LA)
- Estrogen is dominant
- Electromagnetic Field (EMF) Exposure
- Endotoxins— Refined sugars and many starches are more likely to cause intestinal dysbiosis, which can lead to the production of endotoxins.This endotoxin is one of the factors that disrupts mitochondrial function, causing the Warburg effect (cancer metabolism), in which glucose is burned through glycolysis
These all play important roles, but I believe that excess LA and estrogen dominance are the primary causes of mitochondrial dysfunction. This is mainly because LA and estrogen negatively affect your body in similar ways. Both of them:
- Increases free radicals, causing oxidative stress and impairing the mitochondria’s ability to produce energy.
- Increased intracellular calcium uptake leads to increased nitric oxide and superoxide, which increases peroxynitrite and thereby increases oxidative stress.
- Causes an increase in intracellular water, causing the body to retain water.
- Slows metabolic rate and suppresses thyroid gland.
Nearly everyone in the developing world has 10 times more LA in their tissues than their ancestors did 100 years ago. This polyunsaturated fat (PUFA) is highly susceptible to oxidative damage and produces free radicals (such as reactive aldehydes) in the body that damage mitochondria.
These toxic metabolites of LA generate substantial reducing stress due to the accumulation of electrons in the ETC and hinder the forward movement of electrons to complexes IV and V to produce ATP. Furthermore, because LA is embedded in the mitochondrial inner membrane, it can become damaged and leak protons that normally accumulate in the mitochondrial lumen.
This proton gradient is responsible for driving the nanomotor in complex V to generate ATP. These two processes combine to shut down and ultimately prematurely destroy mitochondria. Additionally, when you eat starches, they end up feeding the bacteria in your gut that produce endotoxin, a potent mitochondrial poison.
solution
Finally, if you want to improve or restore mitochondrial function, some key solutions are:
- Reduce your LA intake as much as possible by avoiding processed foods, seed oils, chicken, pork, seeds and nuts.
- Make sure to eat healthy carbs like ripe fruits, raw honey, and maple syrup.
- Decrease lactic acid production and increase carbon dioxide as they have opposite effects.4 You can learn more about this in “The Biology of Carbon Dioxide”.
- Reduce stress, as chronic stress promotes the release of cortisol, a potent inhibitor of mitochondrial function and biogenesis. Progesterone is very helpful here because it is a potent cortisol blocker. You can learn more in What You Need to Know About Estrogen and Serotonin.
- Take supplemental niacinamide because without it, mitochondria cannot produce energy. I recommend taking 50 mg of niacin 3 times a day.
